We developed a series of new reactions taking advantage of the high electron-deficient nature of PFAAs. The F atoms on the phenyl azide lower the LUMO of PFAA, thus the reactions with nucleophiles or dipolarophiles occur at room temperature in high yields without the use of any metal catalysts. These reactions are now been applied to synthesize glyconanomaterials and drug conjugates.
Xie, S.; Lopez, S.; Ramstrom, O.; Yan, M.; Houk, K. N. 1,3-Dipolar Cycloaddition Reactivities of Perfluorinated Aryl Azides with Enamines and Strained Dipolarophiles, (2015) J. Am. Chem. Soc., 137, 2958.
Xie, S.; Zhang, Y.; Ramstrom, O.; Yan, M. Base-catalyzed synthesis of aryl amides from aryl azides and aldehydes, (2016) Chem. Sci., 7, 713.
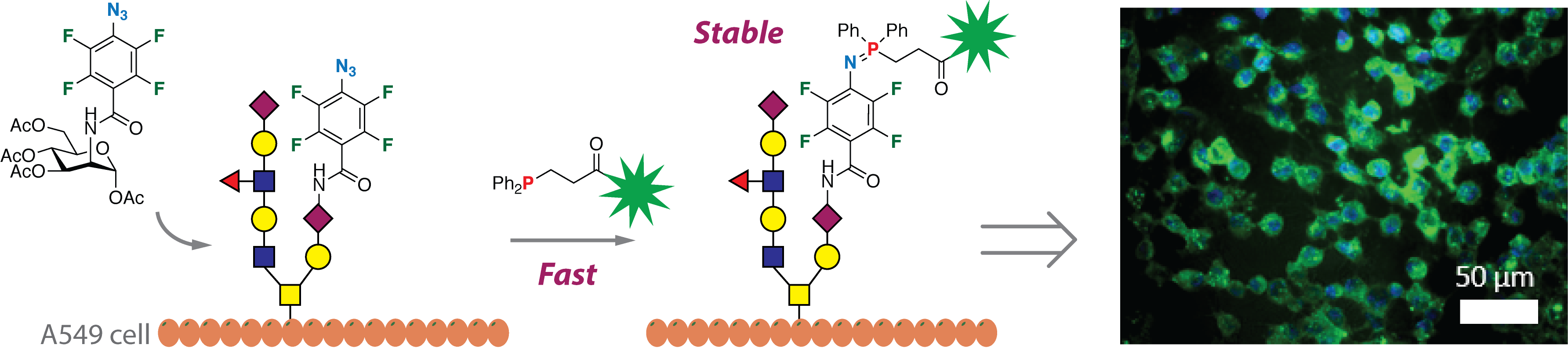
Sundhoro, M.; Jeon, S.; Hao, N.; Park, J.; Ramstrom, O.; Yan, M. Perfluoroaryl Azide–Staudinger Reaction: A Fast and Bioorthogonal Reaction, (2017) Angew. Chem. Int. Ed, 56, 12117.