At UMass Lowell:


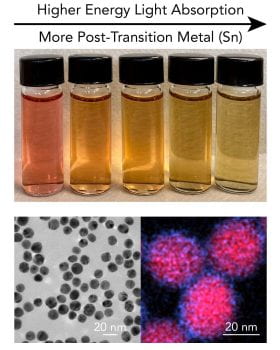

56. Dawes, S.S.; Branco, A.J.; Nagarajan, P.; Jeong, S.; Oluwatosin, B.; Cha, J.H., Sullivan, C.S., Ross, M.B. “Sn Galvanic Exchange Enables Creation of Trimetallic Mixed-Phase Plasmonic Nanoparticles” Submitted ChemRxiv doi: 10.26434/chemrxiv-2025-bcw4w
55. Jeong, S.; Branco, A.J.; Nagarajan, P.; Sullivan, C.S.; Cha, J.H.; Bollen, S.W.; Mason, N.L.; Abeykoon, M.; Olds, D.; Ross, M.B. “Compositional Phase Control in High Entropy Alloys Electrocatalysts Enables Durable Hydrogen Production” Submitted
54. Jeong, S.; Bollen, S.W.; Nagarajan, P.; Ross, M.B. “Capping Agent Selection Enhances Electrocatalytic Water Splitting for High Entropy Alloy Nanoparticles” Submitted
53. Mason, N.L., Branco, A.J., Liu, S., Trancart, M., Jeong, S., Sullivan, C.S., Dawes, S.S., Chatterjee, S., Manukian, S., Hayes, D., Quan, L., Ross, M.B. “Annealing-Driven Phase Control Enables Plasmonic Tunability in Alloy Nanoparticles” Submitted ChemRxiv: doi: 10.26434/chemrxiv-2025-rxqdx
52. Wang, C., Biswas, K., Bello, A., Bello, D.; Ross, M.B. “Surface-Enhanced Raman Spectroscopy Detection of Per- and Poly-Fluoroalkyl Substances in Aqueous Film Forming Foams” Submitted
51. Mirkin, C.A…. Ross, M.B. and 108 others “33 Unresolved Questions in Nanoscience and Nanotechnology” ACS Nano 2025In Press
50. Sullivan, C.S.; Mason, N.L.; Branco, A.J.; Jeong, S.; Ross, M.B. “Size, Composition, and Phase Tunable Plasmonic Absorption in Au-Sn Alloy Nanoparticles” J. Phys. Chem C. 2025 129, 11070-11076.
49. Mason, N.L.; Dawes, S.S.; Vu, D.; Sullivan, C.S.; Chatterjee, S.; Branco, A.J.; Manukian, S.; Hartman, K.M.; Ross, M.B. “Introducing Solid State Chemistry and Nanoscience with Colloidal Au-Sn Alloying” J. Chem. Ed. 2024 101, 3404-3409.
48. Jeong, S.; Branco, A.J.; Bollen, S.W.; Sullivan, C.S.; Ross, M.B. “Universal pH electrocatalytic hydrogen evolution with Au-based high entropy alloys” Nanoscale 2024 16, 11530-11537.
47. Chaurasia, S.; Aravamuthan, S.R.; Sullivan, C.S.; Ross, M.B.; Agar, E. “Investigating manganese- vanadium redox flow batteries for energy storage and subsequent hydrogen generation” ACS Appl. Energy Mater. 2024 7, 11429-11441.
46. Cha, J.H.; Silva, S.M.; Branco, A.J.; Ross, M.B. “Aqueous synthesis of plasmonic gold-tin alloy nanoparticles” J. Vis. Exp. 2024 205, e66628.
45. King, M.E.; Xu, Y.; Nagarajan, P.; Mason, N.L.; Branco, A.J.; Sullivan, C.S.; Silva, S.M.; Jeong, S.; Che, F.; Ross, M.B. “Leveraging bismuth immiscibility to create highly concave noble metal nanoparticles” Chem 2024 6, 1725-1740. ChemRxiv doi: 10.26434/chemrxiv-2023-1644z https://www.cell.com/chem/fulltext/S2451-9294(24)00064-0 Cover Article https://www.cell.com/chem/fulltext/S2451-9294(24)00234-1
44. Sullivan, C.S.; Jeong, S.; King, M.E.; Ross, M.B. “Designing electrocatalysts for hydrogen evolution in saline electrolyte using rapid synthesis on carbon paper supports” Mater. Chem. Front. 2024 8, 1382-1389. ChemRxiv doi:10.26434/chemrxiv-2023-59xhv https://pubs.rsc.org/en/content/articlehtml/2024/qm/d3qm00978e
43. Fonseca Guzman, M.V.; King, M. E.; Mason, N.L.; Sullivan, C.S.; Jeong, S.; Ross, M.B. “Plasmon manipulation by post-transition metal alloying” Matter. 2023 6, 838–854. ChemRxiv doi: 10.26434/chemrxiv-2022-zjdkn https://www.sciencedirect.com/science/article/pii/S2590238523000048 Cover Article https://www.cell.com/issue/S2590-2385(22)X0004-0#fullCover
42. Li, Z.; Wang, S.; Nattermann, U.; Bera, A.K.; Borst, A.J.; Yaman, M.Y.; Bick, M.J.; Yang, E.C.; Sheffler, W.; Lee, B.; Seifert, S.; Hura, G.L.; Nguyen, H.; Kang, A.; Dalal, R; Lubner, J.M.; Hsia, Y.; Haddox, H.; Courbet, A.; Dowling, Q.; Miranda, M.; Favor, A.; Etemadi, A.; Edman, N.I.; Yang, W.; Weidle, C.E.; Sankaran, B.; Negahdari, B.; Ross, M.B.; Ginger, D.S.; Baker, D. “Accurate Computational Design of 3D Protein Crystals” Nat. Mater. 2023 22, 1556-1563.
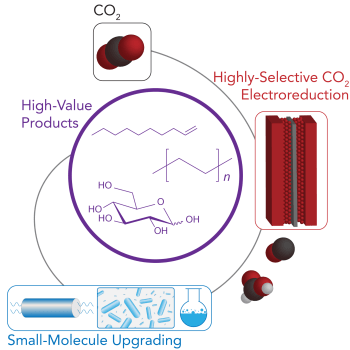
41. Nagarajan, P., Augustine, I. J., Ross, M.B. “Strategies for Multi-Step CO2 Upgrading and Valorization” Cell Rep. Phys. Sci. 2023 4, 101472. https://www.cell.com/cell-reports-physical-science/fulltext/S2666-3864(23)00251-5
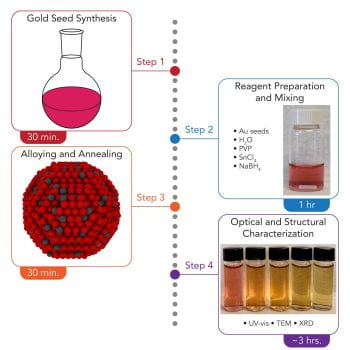
40. Branco, A.J., Dawes, S., Mason, N.L., Fonseca Guzman, M.V., King, M.E., Ross, M.B. “Synthesis of gold-tin alloy nanoparticles with tunable plasmonic properties” STAR Protocols. 2023 4, 102410. https://star-protocols.cell.com/protocols/2799
39. Xu, Y.; Ross, M.B.; Xin, H.; Che, F. “Engineering bimetallic interface and revealing the mechanism for CO2 electroreduction reaction to C3+ liquid chemicals” Cell Rep. Phys. Sci. 2023 4, 101718.
38. Scanga, R.; Sharokhinia, A.; Borges, J.; Ross, M.B.; Reuther, J.F. “Asymmetric polymerization-induced crystallization-driven self assembly of helical, rod-coil poly(aryl isocyanide) block copolymers” J. Am. Chem. Soc. 2023 145, 6319–6329. https://pubs.acs.org/doi/full/10.1021/jacs.2c13354
37. Cestellos-Blanco, S.; Louisia, S.; Ross, M.B.; Li, Y.; Detomasi, T. C.; Cestellos Spradlin, J. N.; Nomura, D. K.; Yang, P. “Toward abiotic sugar synthesis by CO2 electrolysis” Joule 2022 6, 1–20. ChemRxiv doi: 10.26434/chemrxiv-2021-9srsx https://www.sciencedirect.com/science/article/pii/S2542435122004081
36. King, M.E.; Wang, C.; Fonseca Guzman, M.V.; Ross, M.B. “Plasmonics for environmental remediation and pollutant degradation” Chem Catalysis 2022 2, 1–13. https://www.sciencedirect.com/science/article/abs/pii/S2667109322003359
35. Hammerstrom, B.; Niezrecki, C; Hellman, K; Jin, X.; Ross, M.B.; Mack, J. H.; Agar, E.; Trelles, J.P.; Liu, F.; Che, F.;Ryan, D.; Narasimhadevara, M.S.; Usovicz, M. “The viability of implementing hydrogen in the Commonwealth of Massachusetts” Front. Energy Res. 2022 10, 1005101. https://www.frontiersin.org/articles/10.3389/fenrg.2022.1005101
34. King, M.E.; Fonseca Guzman, M.V.; Ross, M.B. “Material strategies for function enhancement in plasmonic architectures” Nanoscale 2021, 14, 602–611. https://pubs.rsc.org/en/content/articlehtml/2022/nr/d1nr06049j
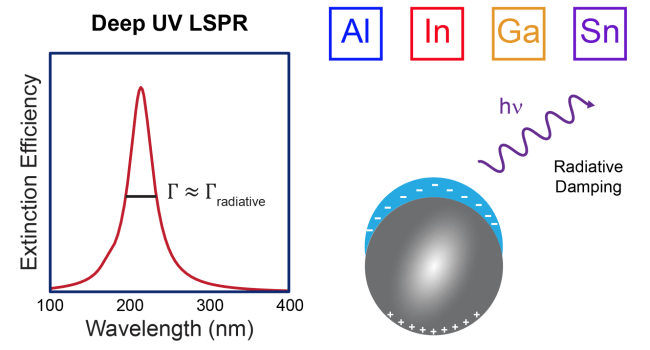
33. Fonseca Guzman, M.V.; Ross, M.B. “Radiative contributions dominate plasmon broadening in post-transition metals in the ultraviolet” J. Phys. Chem. C 2021, 125, 19428–19437. ChemRxiv doi: 10.33774/chemrxiv-2021-pk3k7-v4 https://pubs.acs.org/doi/full/10.1021/acs.jpcc.1c03895
32. Folgueras, M.C.; Jin, J.; Gao, M.; Quan, L.N.; Steele, J.A.; Srivastava, S.; Ross, M.B.; Zhang, R.; Seeler, F.; Schierle-Ardnt, K.; Asta, M.; Yang, P. “Lattice dynamics and optoelectronic properties of zero-dimensional perovskite Cs2TeX6 (X= CL-, Br, I-), single crystals” J. Phys. Chem. C 2021, 125, 25126–25139. https://pubs.acs.org/doi/full/10.1021/acs.jpcc.1c08332
31. Chen, C.; Li, Y.; Yu, S.; Louisia, S.; Jin, J.; Li, M.; Ross, M.B.; Yang, P. “Cu-Ag tandem catalysts for high-rate CO2 electrolysis towards multicarbons” Joule 2020, 4, 1688–1699. https://www.sciencedirect.com/science/article/pii/S2542435120303275
30. Ross, M.B. “Carbon dioxide recycling makes waves” Joule 2019 3, 1814–1816. https://www.sciencedirect.com/science/article/pii/S2542435119303630
Postdoctoral Work:
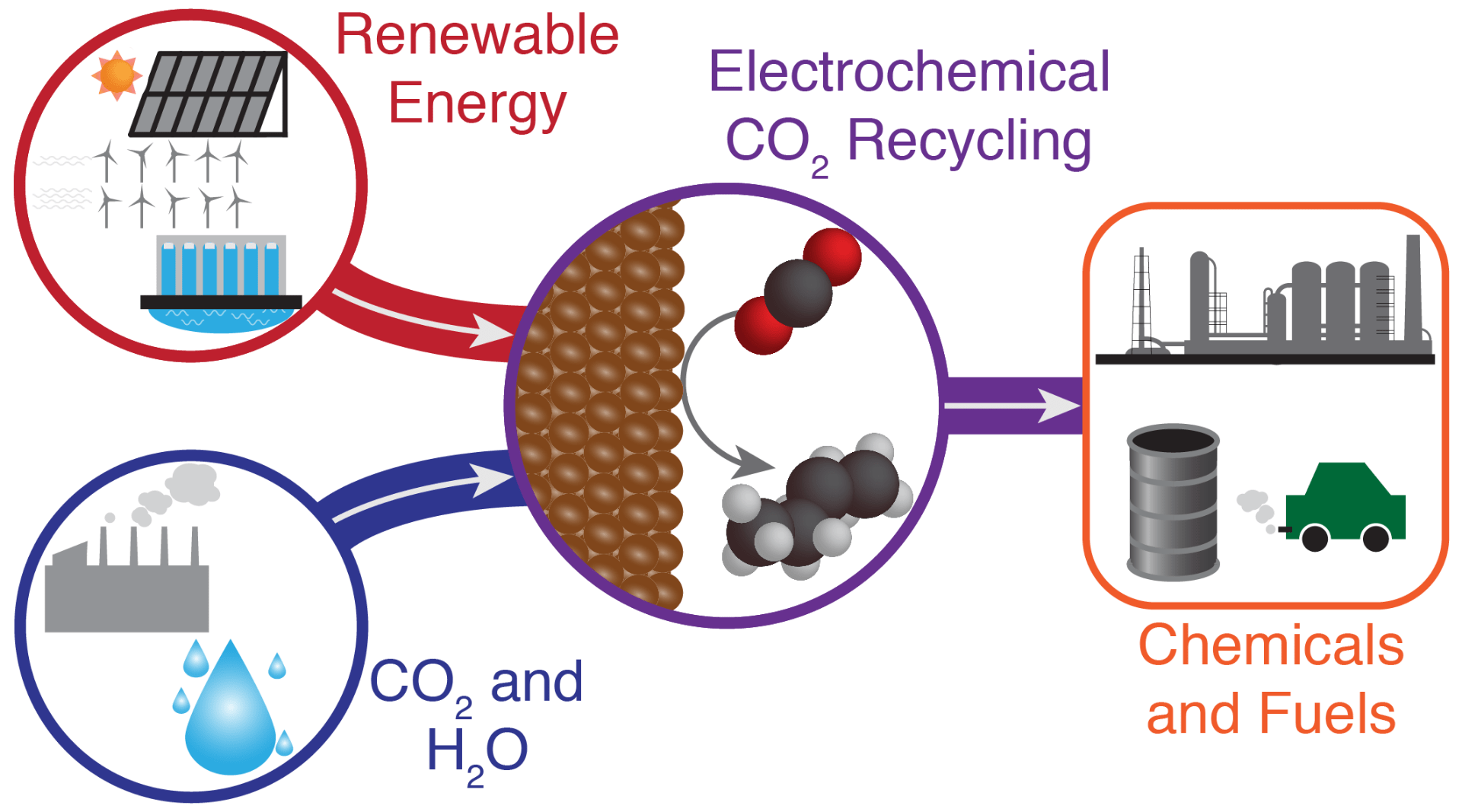
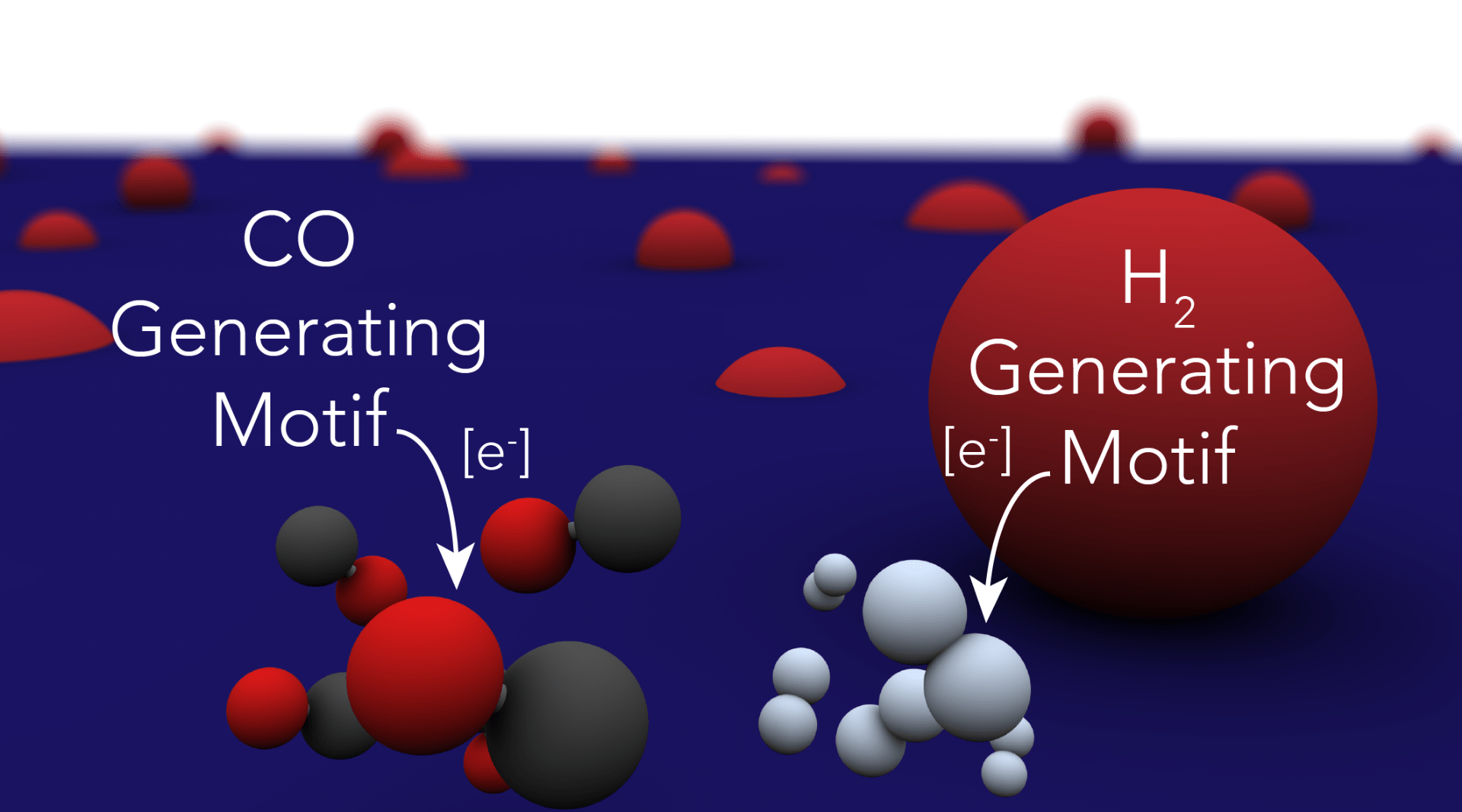
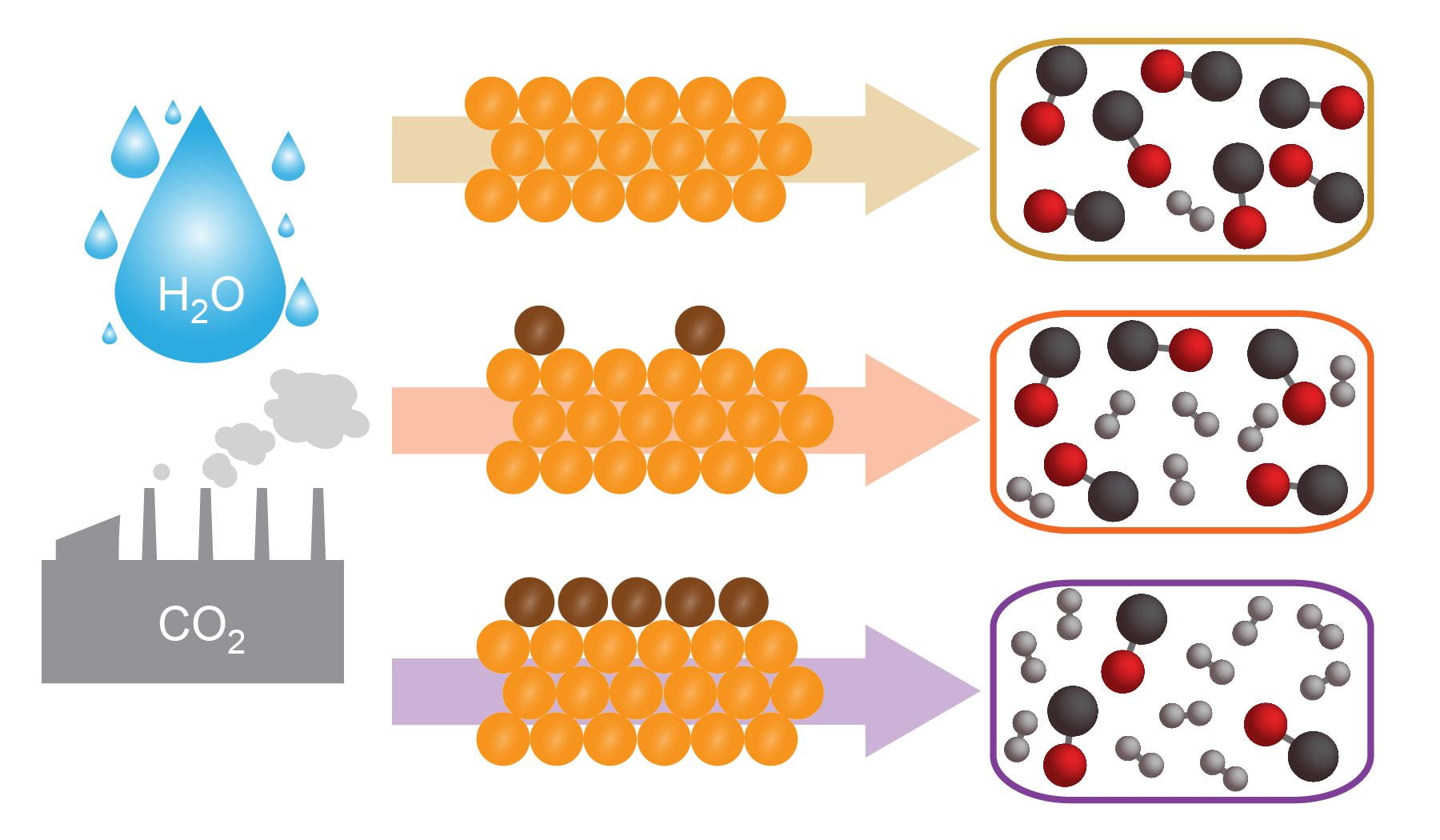
29. Ross, M. B.; De Luna, P.; Li, Y.; Dinh, C. T.; Kim, D.; Yang, P.; Sargent, E. H. “Designing materials for electrocatalytic carbon dioxide recycling” Nat. Catal., 2019, 2, 648–658. https://www.nature.com/articles/s41929-019-0306-7
28. Ross, M.B.; Li, Y.; De Luna, P.; Dinh, C. T.; Kim, D.; Sargent, E. H.; Yang, P. “Electrocatalytic rate alignment enhances syngas generation” Joule. 2019, 3, 1–8. https://www.sciencedirect.com/science/article/pii/S2542435118304513
27. Kim, H. Y.; Ross, M. B.; Kornienko, N.; Zhang, L.; Guo, J.; Kim, D.; Yang, P.; McCloskey, B. D. “Efficient hydrogen peroxide generation using reduced graphene oxide-based oxygen reduction electrocatalysts” Nat. Catal. 2018, 1, 282–289. https://www.nature.com/articles/s41929-018-0044-2
26. De Luna, P.; Quintero–Bermudez, R.; Dinh, C. T.; Ross, M. B.; Bushuyev, O.; Todorovic, P.; Regier, T.; Yang, P.; Sargent, E. H. “Catalyst electro–redeposition catalysts controls morphology and oxidation state for selective carbon dioxide reduction” Nat. Catal. 2018, 1, 103–110. https://www.nature.com/articles/s41929-017-0018-9
25. Kibria, M.D.; Dinh, C. T.; Seifitokaldahni, A.; De Luna, P.; Burdyny, T.; Quintero–Bermudez, R.; Ross, M. B.; Bushuyev, O.S.; Garcia de Arquer, F.P.; Yang, P.; Sinton, D.; Sargent, E. H. “A Surface Reconstruction Route to High Productivity and Selectivity in CO2 Reduction toward C2+ Hydrocarbons” Adv. Mater, 2018, 30, 1804867. https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201804867
24. Ross, M. B.; Dinh, C. T.; Li, Y.; Kim, D.; De Luna, P.; Sargent, E. H.; Yang, P. “Tunable Cu–enrichment enables designer syngas electrosynthesis from CO2” J. Am. Chem. Soc. 2017, 139, 9359–9363. https://pubs.acs.org/doi/full/10.1021/jacs.7b04892
23. Li, Y.*; Cui, F.*; Ross, M. B.; Kim, D.; Sun, Y.; Yang, P. “Structure–sensitive CO2 electroreduction to hydrocarbons on ultrathin five–fold twinned copper nanowires” Nano Lett. 2017, 17, 1312–1317. https://pubs.acs.org/doi/full/10.1021/acs.nanolett.6b05287
22. Zheng, X.*; De Luna, P.*; Garcia de Arquer, F. P.; Zhang, B.; Becknell, N.; Ross, M. B., et al. “Sulfur modulated tin sites enable efficient electrochemical reduction of CO2 to formate” Joule, 2017, 1, 794–85. https://www.sciencedirect.com/science/article/pii/S2542435117300880
Graduate Work:
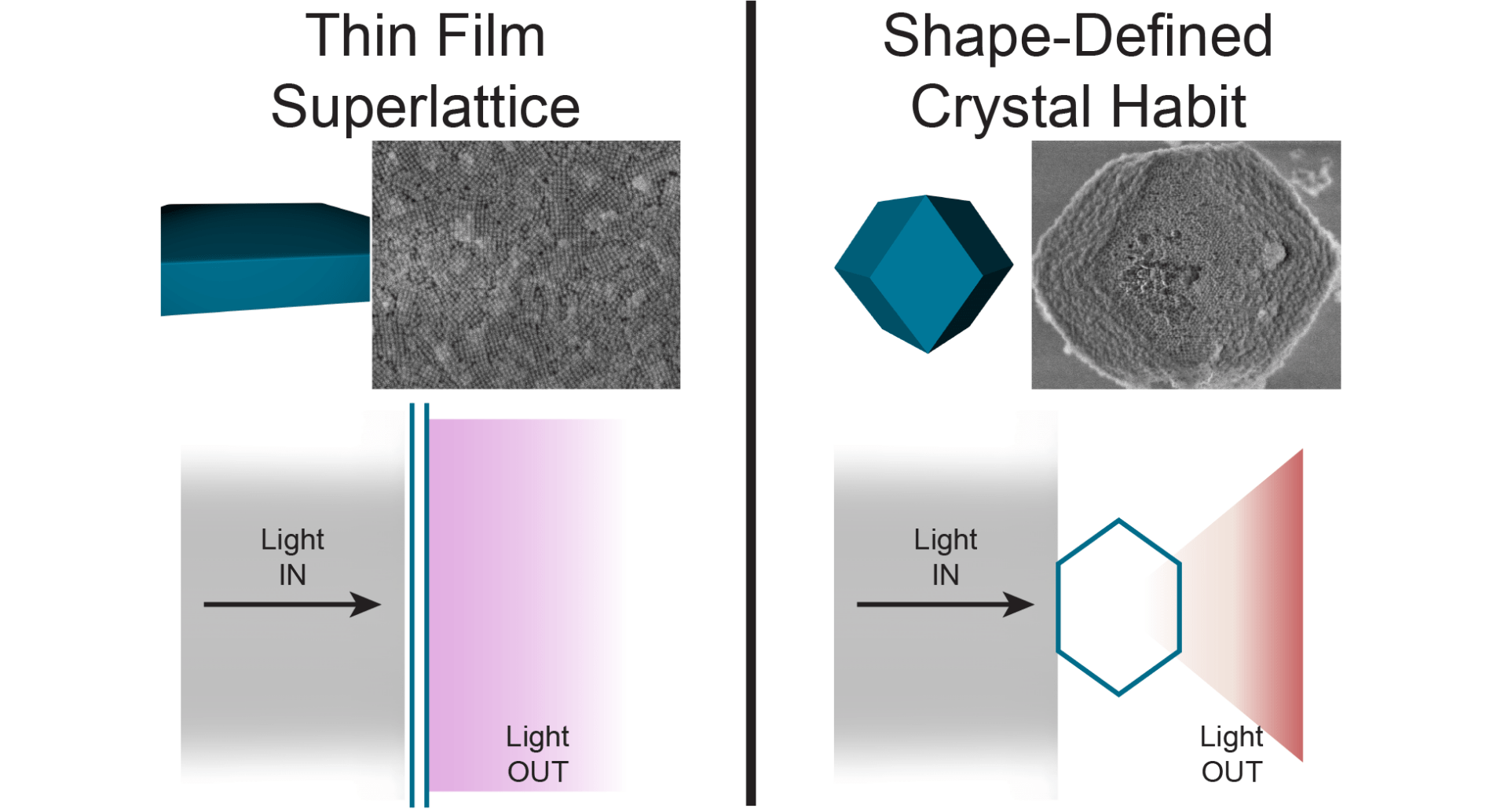
21. Ashley, M. J.; Bourgeois, M. R.; Murthy, R. R.; Laramy, C. R.; Ross, M. B.; Naik, R.R.; Schatz, G. C.; Mirkin, C. A. “Shape and size control of substrate–grown gold nanoparticles for surface–enhanced Raman spectroscopy detection of chemical analytes” J. Phys. Chem. C. 2018, 122, 2307–2314. https://pubs.acs.org/doi/full/10.1021/acs.jpcc.7b11440
20. Wang, S.; McGuirk, C. M.; Ross, M. B.; Wang, S.; Chen, P. C.; Xing, H.; Liu, Y.; Mirkin, C. A. “General and direct method for preparing oligonucleotide–functionalized metal–organic framework nanoparticles” J. Am. Chem. Soc. 2017, 139, 9827–9830. https://pubs.acs.org/doi/full/10.1021/jacs.7b05633
19. Bourgeois, M. R.*; Liu, A. T.*; Ross, M. B.; Berlin, J. M.; Schatz, G. C. “Self–assembled plasmonic metamolecules exhibiting tunable magnetic response at optical frequencies” J. Phys. Chem. C 2017, 121, 15915–15921. https://pubs.acs.org/doi/full/10.1021/acs.jpcc.7b03817
18. Sun, L.*; Lin, H.*; Park, D. J.*; Bourgeois, M. R.; Ross, M. B.; Ku, J. C.; Schatz. G. C; Mirkin, C. A. “Polarization–dependent optical response in anisotropic nanoparticle–DNA superlattices” Nano Lett. 2017, 17, 2313–2318. https://pubs.acs.org/doi/full/10.1021/acs.nanolett.6b05101
17. Ross, M. B.; Ku, J. C.; Lee, B.; Mirkin, C. A.; Schatz, G. C., “Plasmonic metallurgy enabled by DNA” Adv. Mater. 2016, 28, 2790–2794. https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201505806
16. Ross, M. B.; Mirkin, C. A.; Schatz, G. C. “Optical properties of one–, two–, and three–dimensional arrays of plasmonic nanostructures” J. Phys. Chem. C 2016, 2, 816–830. https://pubs.acs.org/doi/full/10.1021/acs.jpcc.5b10800
15. Ross, M. B.*; Bourgeois, M. R.*; Mirkin, C. A.; Schatz, G. C. “Magneto–optical response of cobalt interacting with plasmonic nanoparticle superlattices” J. Phys. Chem. Lett. 2016, 7, 4732–4738. https://pubs.acs.org/doi/full/10.1021/acs.jpclett.6b02259
14. Ross, M. B.; Ashley, M. J.; Schmucker, A. L.; Singamaneni, S.; Naik, R.; Schatz, G. C.; Mirkin, C. A. “Structure–function relationships in SERS–active plasmonic paper” J. Phys. Chem. C 2016, 120, 20789–20797. https://pubs.acs.org/doi/full/10.1021/acs.jpcc.6b02019
13. Barnaby, S. N.; Ross, M. B.; Thaner, R. V.; Lee, B.; Schatz, G. C.; Mirkin, C. A. “Enzymatically controlled vacancies in nanoparticle crystals” Nano Lett. 2016, 16, 5114–5119. https://pubs.acs.org/doi/full/10.1021/acs.nanolett.6b02042
12. Sharma, B.; Cardinal, M. F.; Ross, M. B.; Zrimsek, A.; Bykov, S.; Punihaole, D.; Asher, A.; Schatz, G. C.; Van Duyne, R. P. “Aluminum film–over–nanosphere substrates for deep–UV surface–enhanced resonance Raman spectroscopy” Nano. Lett. 2016, 16, 7968–7973. https://pubs.acs.org/doi/full/10.1021/acs.nanolett.6b04296
11. Ashley, M. J.; O’Brien, M. N.; Hedderick, K. R.; Mason, J. A.; Ross, M. B.; Mirkin, C. A. “Templated synthesis of uniform perovskite nanowire arrays” J. Am. Chem. Soc. 2016, 138, 10096–10099. https://pubs.acs.org/doi/full/10.1021/jacs.6b05901
10. Ross, M. B.; Ku, J. C.; Vaccarezza, V. M.; Schatz, G. C.; Mirkin, C. A. “Nanoscale form dictates mesoscale function in plasmonic DNA nanoparticle superlattices” Nat. Nanotechnol. 2015, 10, 453–458. https://www.nature.com/articles/nnano.2015.68
9. Ross, M. B.; Ku, J. C.; Blaber, M. G.; Mirkin, C. A.; Schatz, G.C. “Defect tolerance and the effect of structural inhomogeneity in plasmonic DNA–nanoparticle superlattices” Proc. Natl. Acad. Sci. U.S.A. 2015, 112, 10292–10297. https://www.pnas.org/doi/abs/10.1073/pnas.1513058112
8. Ross, M. B. & Schatz, G. C. “Radiative effects in plasmonic aluminum and silver nanospheres and nanorods” J. Phys. D: Appl. Phys. 2015, 48, 184004. https://iopscience.iop.org/article/10.1088/0022-3727/48/18/184004/
7. Ku, J. C.; Ross, M. B.; Schatz, G. C.; Mirkin, C. A. “Conformable, macroscopic crystalline nanoparticle sheets assembled with DNA” Adv. Mater. 2015, 27, 3159–3163. https://onlinelibrary.wiley.com/doi/10.1002/adma.201500858
6. Barnaby, S. N.; Thaner, R. V.; Ross, M. B.; Brown, K. A.; Schatz, G. C.; Mirkin C. A. “Modular and chemically responsive oligonucleotide bonds in nanoparticle superlattices” J. Am. Chem. Soc. 2015, 137, 13566–13571. https://pubs.acs.org/doi/full/10.1021/jacs.5b07908
5. Ozel, T.*; Ashley, M. J.*; Bourret, G. R.; Ross, M. B.; Schatz, G. C.; Mirkin, C. A. “Solution–dispersible metal nanorings with deliberately controllable compositions and architectural parameters” Nano Lett. 2015, 15, 5273–5278. https://pubs.acs.org/doi/full/10.1021/acs.nanolett.5b01594
4. Lin, Q. Y.*; Li, Z.*; Brown, K. A.; O’Brien, M. N.; Ross, M. B.; Zhou, Y.; Butun, S.; Chen, P. C.; Schatz, G. C.; Dravid, V. P.; Aydin, K.; Mirkin, C. A. “Strong coupling between plasmonic gap modes and photonic lattice modes in DNA–assembled gold nanocube arrays” Nano Lett. 2015, 15, 4699–4703. https://pubs.acs.org/doi/full/10.1021/acs.nanolett.5b01548
3. Ross, M. B.; Blaber, M. G.; Schatz, G. C. “Using nanoscale and mesoscale anisotropy to engineer the optical response of three–dimensional plasmonic metamaterials” Nat. Commun. 2014, 5, 4090. https://www.nature.com/articles/ncomms5090
2. Ross, M. B. & Schatz, G. C. “Aluminum and indium plasmonic nanoantennas in the ultraviolet” J. Phys. Chem. C. 2014, 118, 12506–12514. https://pubs.acs.org/doi/full/10.1021/jp503323u
1. Young, K. Y.*; Ross, M. B.*; Blaber, M. G.; Rycenga, M.; Jones, M. R.; Zhang, C.; Senesi, A. J.; Lee, B.; Schatz, G. C.; Mirkin, C. A. “Using DNA to design plasmonic metamaterials with tunable optical properties” Adv. Mater. 2014, 26, 653–659. https://onlinelibrary.wiley.com/doi/full/10.1002/adma.201302938

