Microbial fermentation for production of fuels, chemical, nutraceuticals, and pharmaceuticals has started to show great advantages over the traditional petrochemical manufacturing process due to the use of renewable feedstocks, less environmental footprint, and operation with much milder reaction conditions. Advances in modern biotechnology such as synthetic biology, molecular biology, and bioinformatics makes the biomanufacturing a more attractive option. At the same time, most of current biomanufacturing facilities were still built as bath/fed-batch operating systems. However, frequent down time and inability of keeping an optimum production rate significantly limit the productivity, leading to high capital and operating costs. This old-style fermentation technology developed from 1950s is far behind the fast pace of modern biotechnology in other technical areas, which makes it hard to compete with the fossil oil-based chemical manufacturing processes. Continuous fermentation has been studied in the past decades, but four major challenges still remain before it becomes a standard practice at industrial scales:
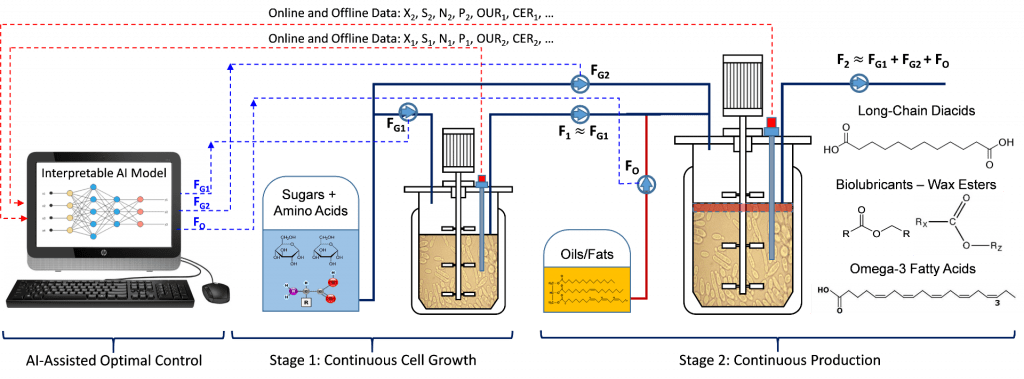
To overcome the challenges above, an ideal continuous biomanufacturing system should (1) use a high-producing strain with high genetic stability, (2) be resistant to contamination or at low risks of contamination, (3) decouple cell growth and product formation to maximize the overall titer, rate, and yield, and (4) be equipped with intelligent process control strategies that can be applied to different fermentation products.

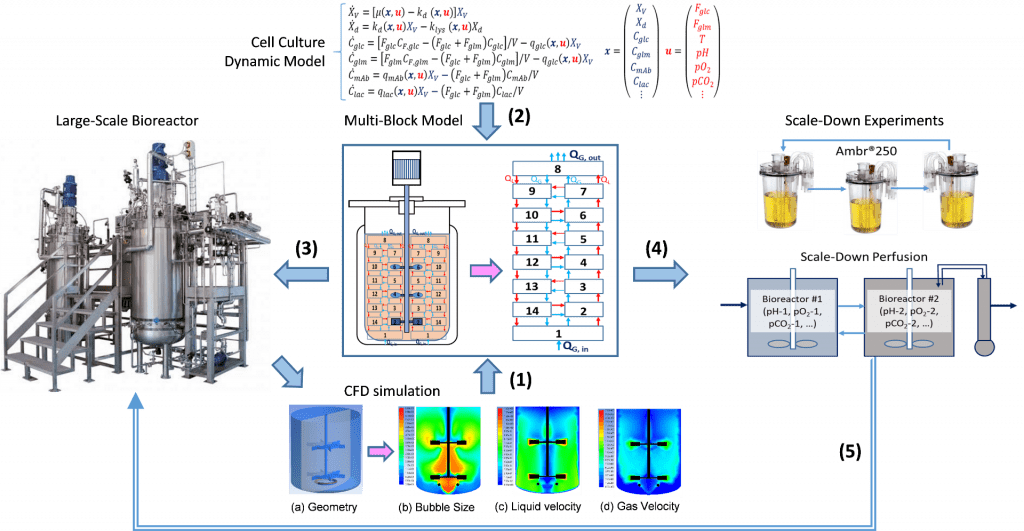
Modeling will play a critically important role in my industrial biotechnology research platform. Mathematical models will be built to analyze, predict, optimize, and control the fermentation processes for each specific product. Different from the traditional kinetic models, metabolic flux analysis, mixing and mass transfer study results, together with the available fermentation data from bench to large scale will be used to build and validate my platform-based model. The model will be firstly used to choose and design a batch, fed-batch, or continuous fermentation process suitable for each specific product, based on the simulation results and economic analysis. After that, the model will be further used to optimize the designed fermentation process. Dynamic optimization principles (e.g. optimal control strategy) will be applied to maximize the titer, rate, and yield of the targeted product and minimize the cost of manufacturing (COM). At last, the model will be used to help scale-up the bioprocess by simulating and analyzing the fermentation performance under large-scale bioreactor conditions. In addition to the first principal models of cell growth, substrate consumption, and product formation, artificial intelligence (AI) may also be integrated in bioprocess modeling, optimization, and control.
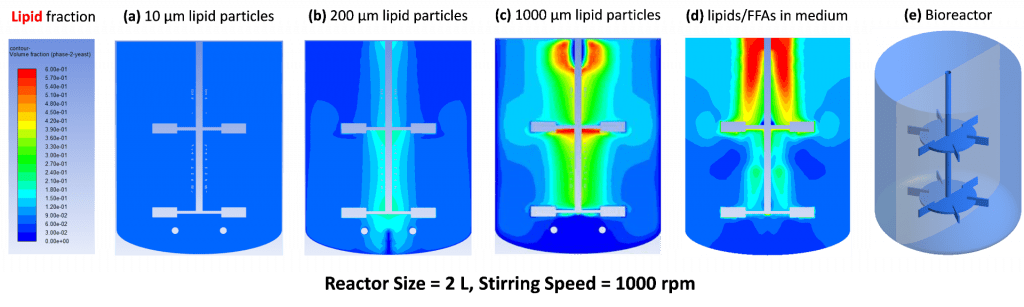
Computational Fluid Dynamics (CFD) provides detailed simulation of hydrodynamics, mixing, and mass transfer in a bioreactor that cannot be easily measured by experiments. The bioreactor system consists of multiple phases (gas, aqueous medium, cells, and organic phase such as lipid droplets) with changes due to bioreactions. We plan to first conduct CFD simulation for multi-phase physical behaviors. After that, bioreactions including cell growth, nitrogen consumption, oxygen uptake, carbon source (such as glucose) consumption, CO2 evolution, and product formation will be integrated in the overall bioreactor simulations. In future, we hope the CFD can be upgraded to simulate the intracellular biochemical responses to bioreactor local environment. Also, cell-to-cell communications and interactions may fit to our further interests.
Current biomanufacturing process is still far below its maximum potential due to the limitations in bioreactor’s capability and the sensitivity of microbial or mammalian cells to the stresses in a bioreactor environment. This issue becomes more severe in commercial-scale bioreactors due to the inhomogeneous mixing and mass transfer, especially for biomanufacturing under high cell density conditions. For example, the typical mixing time in a lab-scale bioreactor is within seconds, while this may increase to 2-3 minutes in a commercial scale bioreactor. The limitations in mixing and mass transfer may lead to significantly underperformed biomanufacturing at large scales. To mitigate the scale-up risks and understand better the effect of key process variables on cell growth and the product formation at large scales, we aim to provide a novel and general scale-down technology that will use a new multi-block scale-down model to reveal the limitations and inhomogeneity in large scale bioreactors, and then to further use the model-guided scale-down experiments and improve large-scale bioreactor design and operations.
Specifically, a large-size bioreactor vessel is approximated by a reaction system consisting of multiple interconnected blocks (compartments) with each block assumed homogenous condition by averaging individual variables in it. The average gas and liquid flows and mass transfer coefficients in each block are predetermined by computational fluid dynamics (CFD) simulations. Cell culture and fermentation model will then be applied to the multi-block reaction system to simulate the overall bioreactor performance. This scale-down technology platform will eventually lead to significantly improved productivity with consistent quality at large scales.