Our research focuses on system engineering in life sciences. It covers Process Analytical Technology (PAT) and Quality By Design (QbD), Application of experimental design (DoE) and multivariate data analysis (MVDA), Supply chain management in biologics, and Chemometrics in life sciences. We are aiming at developing innovative systems technology that can improve drug development efficiency and manufacturing productivity, and developing transformative diagnostic systems and tools for selected diseases with chemometrics framework. Integration of medical devices with multivariate statistical method is also being explored to develop practical diagnostic tools.
A. AMBIC Advanced Manufacturing Platform for Adeno-Associated Virus (AAV) Production
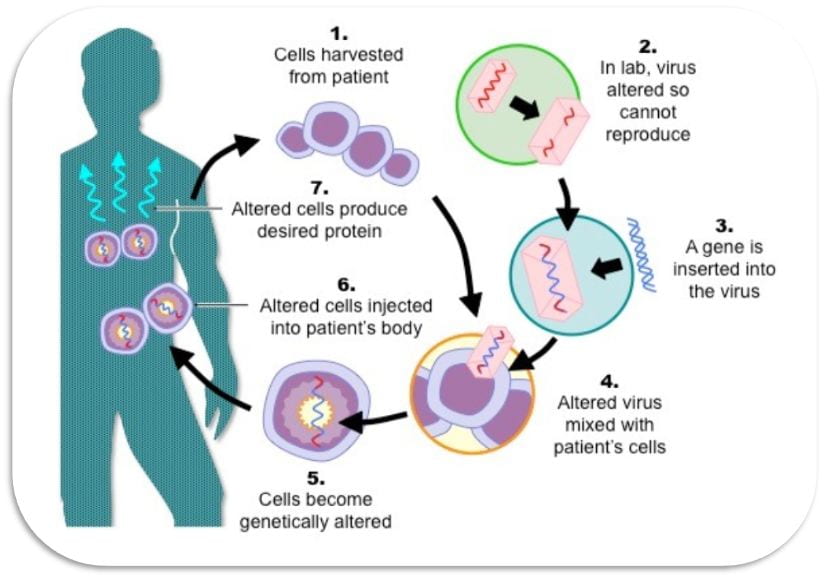
Gene therapy products are an exciting and transformative class of biotherapeutics. Clinical and preclinical data has shown immense promise for dozens of gene therapy products. The processes that are currently used to produce viral vectors are simply scaled-up versions of laboratory-scale procedures, which are time consuming, have low reproducibility, result in low productivity and product recovery, and are expensive. The proposed project aims to develop a next-generation modular process plant utilizing bioprocess engineering approaches for high productivity, robust operation, and drug product attribute control. The platform will be continuous, modular, closed loop controlled process that require a small footprint (compact facilities) and minimize labor. These robust and efficient processes will allow for rapid response and rapid turnaround of viral vector requests.
B. Continuous and Semi-Continuous Viral Vaccine Production
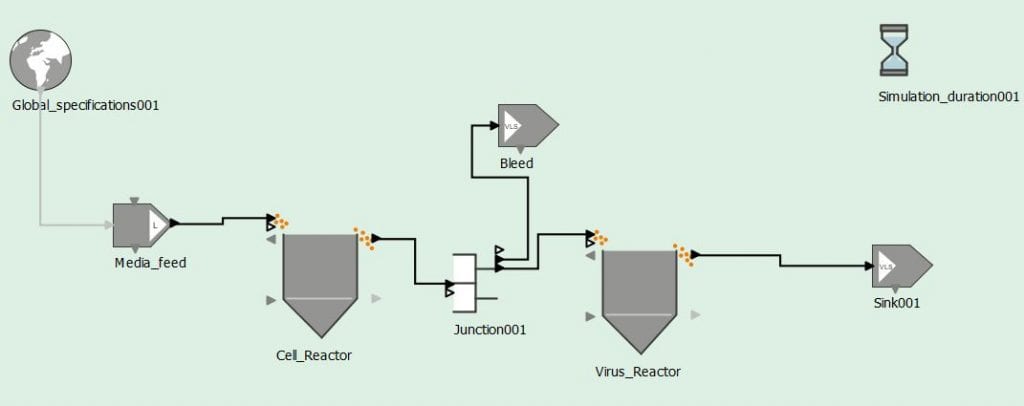
The objective of this project is to establish a continuous and a semi-continuous viral vaccine manufacturing platform. This goal will be accomplished in several phases. Phase I will be based upon first principle modeling efforts and flow sheet modeling to establish the expected results of the platform via simulation. Phase II will involve setting up a small-scale continuous platform. This will be performed at UMass Lowell utilizing viruses that have low reactivities. Finally, Phase III will involve setting up a small-scale semi-continuous platform. Throughout phase II and phase III, critical quality control attributes will be established and monitored. Furthermore, process optimization will occur throughout this entire project utilizing the established model and through improving that same model. The below figure shows a preliminary screenshot of the continuous production flow sheet model.
C. mAb Productivity Improvement by Metabolic Engineering of Novel Inhibitory Biomarker
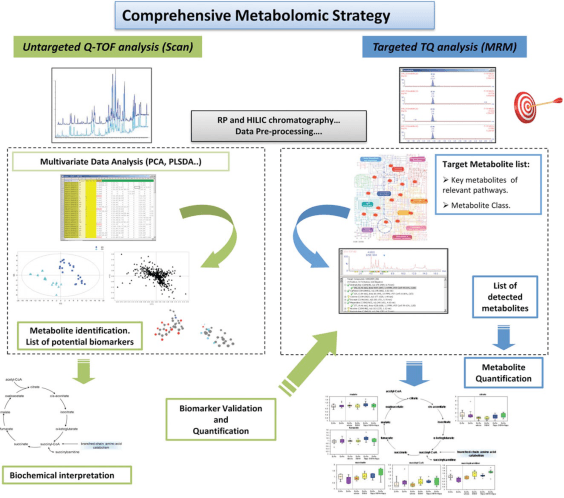
University of Massachusetts Lowell in collaboration with partner organizations – Genentech, Celgene, and Immunogen, propose to advance protein therapeutic productivity by metabolic engineering of novel inhibitory. We are looking to develop a strategy to control metabolite waste within the tolerance along with manipulate perfusion rate, media formulation improvement, and corresponding cell-line development. Successful completion of this proposed effort and application of the resulting cell-culture media formulation and cell-lines will advance the current industry average productivity, reduce the occurrence of shortages of protein therapies and reduce the opportunity costs associated with sub-optimal productivity.
D. Mapping CHO metabolic pathway for AMBIC media formulation optimization
Understanding the metabolism of CHO cell is important to find the strategies for improving the protein production and the efficiency of media utilization. The current commonly used DOEs method is still time-and cost-consuming thus raises the demand of a new efficient approach for media optimization. In this proposal, we will develop a new approach of media optimization by mapping CHO metabolic pathway. Based on identified targets for media optimization from pathway analysis, model simulation will be carried out to in silico validate the optimization strategies and, finally, the new experiments will be designed to verify the modeling hypothesis.
E. Control and Estimation of Glycosylation Profile via Media Supplementation Based on Intracellular First Principle Models in Mammalian Cell Cultures
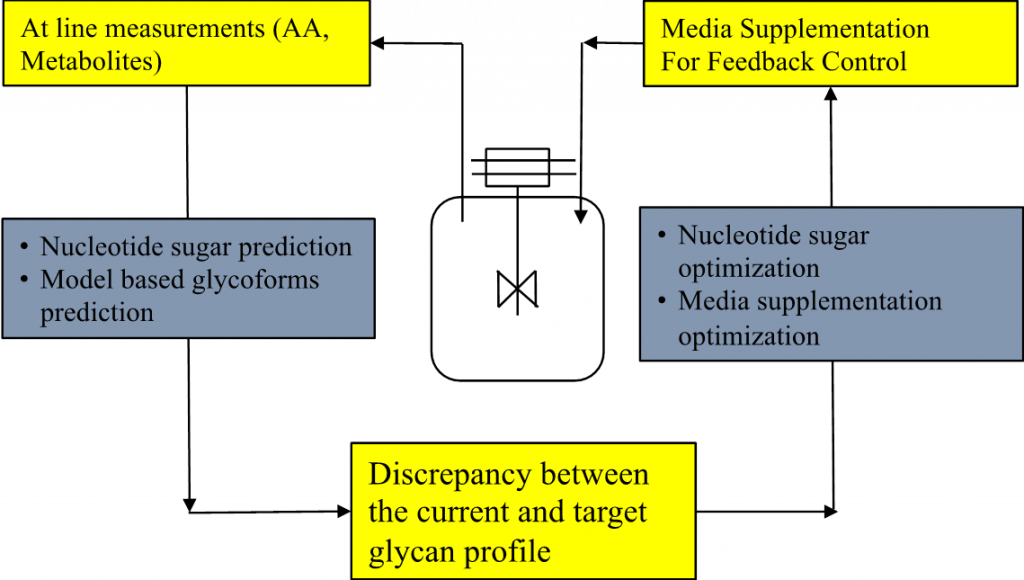
The research objective is to develop robust glycosylation profile predictions and control strategies using mathematical models for mammalian cell-culture products. Critically, systems biology approaches along with experimental studies will be exploited to trace the cellular activity leading to the variations of glycan profiles, and a mechanistic understanding will be used to refine the model. The predictive power of the model will be iteratively improved with culture data from variant culture platforms and cell lines. Our approach should lead to a new class of tools and knowledge for glycosylation profiling and control beyond existing technologies, thus having a transformative impact on academia and the biopharmaceutical industry.
F. Characterization of Media Precipitate and Determination of Precipitation Kinetics
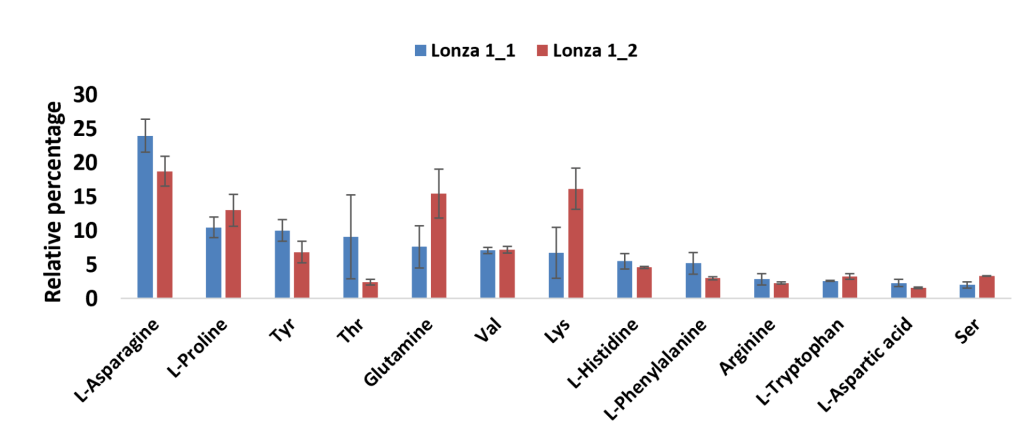
Process intensification of mAb production is leading to more concentrated feed media causing issues with precipitation of solids from the media solution. This results in processing problems since components in the precipitate are no longer in solution and bioavailable, this changes the media composition leading to variability in cell culture performance. The goal of this work is to characterize the feed media precipitate, and in particular to identify the components so that mitigation strategies can be developed. The precipitate was predominately found to be organic, and was analyzed with LC-MS (Liquid Chromatography – Mass Spectrometry) to identify the constituent components. Up to 16 amino acids were identified with tyrosine, histidine, arginine and phenylalanine being the most prevalent amino acids. Elemental analysis with ICP-OES (Inductively Coupled Plasma – Optical Emission Spectroscopy) and XRD (X-Ray Diffraction) suggest that metal phosphates are the predominant inorganic components. Precipitation experiments informed by this analysis are being used to determine the formation kinetics, which in turn can be used to better design feed media and better process scheduling. This work is critical for the current trends of process intensification for mAb production to continue.
