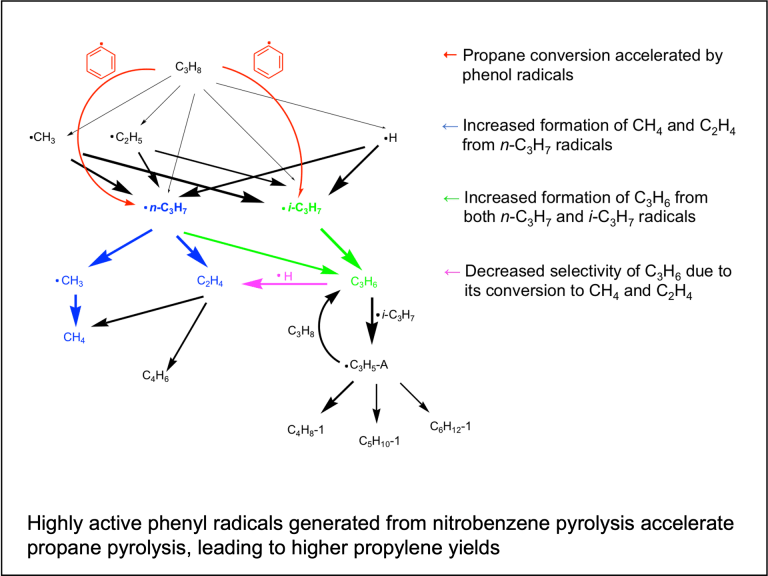
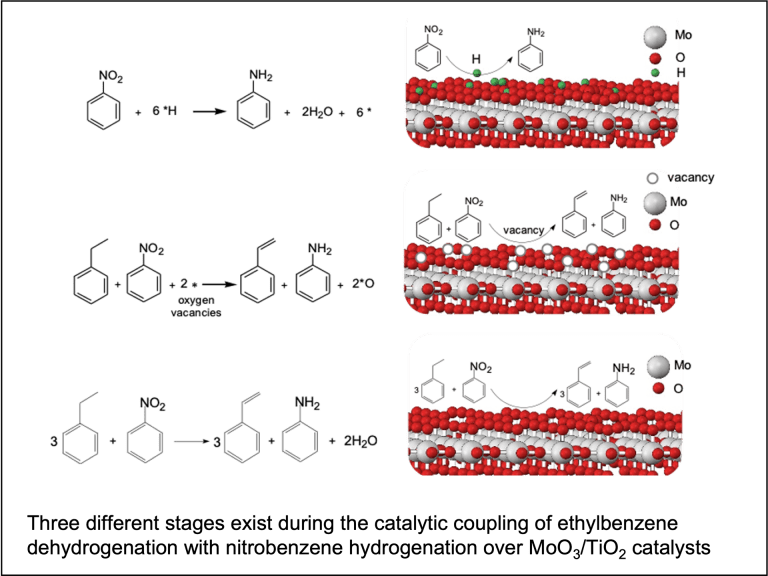
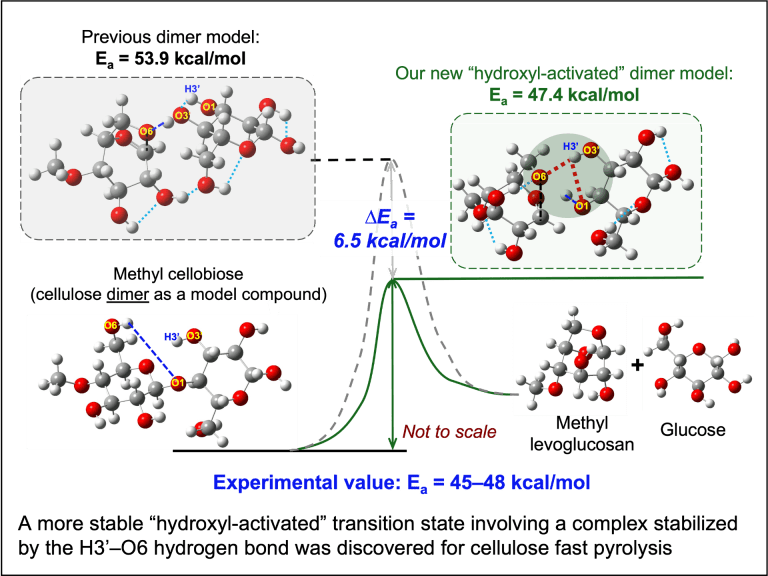
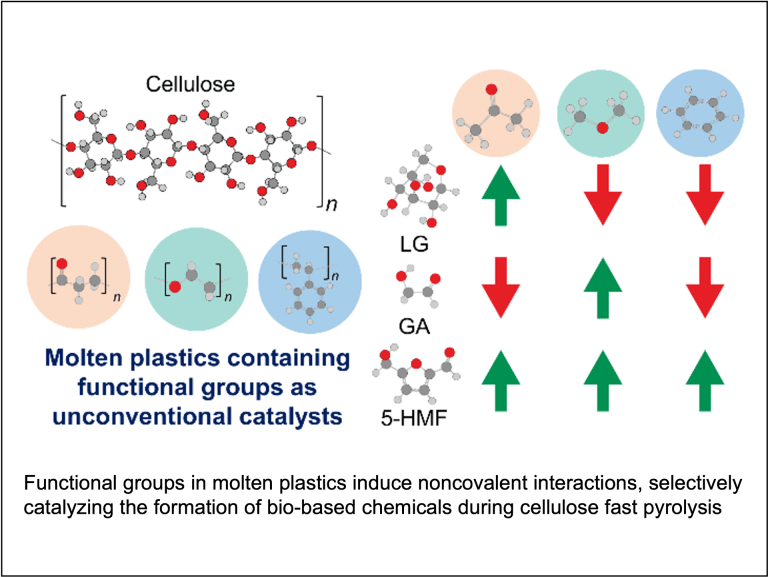
Copyrolysis of waste mixtures
Typical waste components, such as biomass, plastics, and food waste, are immiscible under pyrolysis conditions. They also possess drastically different degradation timescales. Our group seeks to understand the interplay between chemical kinetics and mass transfer during copyrolysis of waste components so that the entire pyrolysis process can be flexibly tuned and manipulated to produce high yields of target chemicals.
Upcycling of single-use multi-layer plastic films via an integrated chemolytic delamination and plasma carbonization process (collaborated with Prof. Grace Chen, Plastics Engineering and Prof. Juan Pablo Trelles, Mechanical Engineering)
More than 100 million tons of multi-layer plastic films are produced globally every year, mostly used for packaging. The heterogeneity of the films prohibits the use of traditional plastic recycling techniques. In this project, we are exploring an integrated process to delaminate the film and convert the condensation polymers into monomers first, followed by a nonthermal plasma process to carbonize the substrate into carbon and hydrogen gas.
Bioconversion of polyester wastes (collaborated with Prof. Margaret Sobkowicz-Kline, Plastics Engineering, Prof. Dongming Xie, Chemical Engineering, and Dr. Gregg Beckham, National Renewable Energy Laboratory)
Plastics wastes has gained increased attention due to its negative impacts on the environment. Most plastic recycling methods have significant energy requirements or limitations. Our group seeks to use reactor design principles to develop biochemical processes for the energy efficient and economical viable recycling of heterogeneous polyester wastes.
Low-temperature plasma-assisted depolymerization of solid waste (collaborated with Profs. Juan Pablo Trelles and Hunter Mack, Mechanical Engineering)
Typical thermochemical depolymerization methods either require high temperature (pyrolysis) or high pressure (hydrothermal liquefaction). The use of low-temperature, non-equilibrium plasma on solid waste decomposition presents distinct advantages compared to these traditional thermochemical methods. Particularly, it allows direct use of electricity from renewable resources, provides high energy density, and poses potential for greater conversion, selectivity, and energy efficiency. Our group seeks to derive fundamental physical–chemical mechanisms for the production of value-added chemicals from solid waste feedstock (plastics, biomass, etc.) using atmospheric pressure non-thermal plasma.
Production of fuel additives from sawdust biomass wastes (collaborated with Prof. Hunter Mack, Mechanical Engineering and Profs. Schwartz and Wheeler, Chemical Engineering at the University of Maine)
Sawmills and other forest industry operations produce a large amount of biomass waste. Our group seeks to discover reaction pathways leading to new biofuel formulations that will have improved fuel properties. The manipulation of reaction pathways are achieved with different pretreatment methods and new catalytic upgrading strategies.
Safer solvents for the purification of active pharmaceutical ingredients (collaborated with Prof. Gregory Morose, Toxics Use Reduction Institute)
Column chromatography is a common technique to separate active pharmaceutical ingredients (APIs) from impurities for product purification in pharmaceutical manufacturing. The operation typically involves the use of toxic organic solvents, most notably dichloromethane, as the mobile phase. The objective of this project is to identify and test safer solvents or solvent blends as alternatives for API purification using column chromatography.
Catalytic reaction coupling (collaborated with Prof. Zhiyong Gu, Chemical Engineering)
Many industrially relevant chemical reactions are thermodynamically limited to equilibrium conversions in which high yields of target products are difficult to achieve. Our group explores coupling of such reactions with a separate reaction that consumes one or more of the products so that the equilibrium can be shifted. The key is to design an effective catalyst that lowers the kinetic barrier and facilitates such reaction coupling.
Safer solvents for liquid chromatography applications (collaborated with Prof. Gregory Morose, Toxics Use Reduction Institute)
Liquid chromatography (LC) is a technique widely used in pharmaceutical, medical, food, dye, or environmental analysis industries to identify and quantify organic compounds in a complex mixture. Typical operation of LC involves continuous use of solvents to dissolve and separate the analytes. The most common solvents for HPLC applications are methanol, acetonitrile, and tetrahydrofuran, all of which pose environmental concerns. Our group seeks to use Hansen Solubility Parameters in Practice (HSPiP) to identify green solvents or solvent blends as possible replacements for existing LC solvents.
Pyrolysis chemistry of thermal protection system ablative materials
Thermal Protection Systems (TPS) ablative materials are used to protect space vehicles during re-etry when the ablation process dramatically heats up the vehicle surfaces. One of the critical steps in ablative material development is to provide state-of-the-art material-response models to ensure optimal heat-shield design. In-depth knowledge of the pyrolysis kinetics of the virgin TPS materials exposed to a high-temperature, low-pressure environment is essential for an accurate model. Our group is using novel custom-made reactor setups to investigate the reaction kinetics and species production from the pyrolysis of TPS materials.
We are grateful for the funding support from the following sponsors:

